Moving the Needle on Dementia Care
Nov 07, 2022
By Sanjay Khurana, Investor-in-Residence, Techstars Future of Longevity Accelerator
Estimated reading time: 13-minutes
As someone “in the biz”, I thought my experience in caregiving and healthcare prepared me for what lay ahead for my wife and me as we started to care for my mother-in-law who had dementia. Oh boy – was I delusional or what?
Nothing prepared us for the roller coaster of dealing with dementia. While we were fortunate to afford the care she needed in the comfort of our home, adjusting our work and life around her needs was challenging, to say the least. The journey seemed long and arduous, emotionally draining, and often confusing and illogical. We witnessed her loss of memory and executive function, sleep deprivation, delirium, aggression, agitation, restlessness, frequent falls, isolation, lack of appetite, loss of bodily function, and eventually passing from complications in the emergency room. As I reflect on our experience, it leaves me with two thoughts - how does someone without means or family support provide quality care at home? If we hacked our way through this journey, is everyone doing the same? That brings me to the purpose of this blog. We need innovative, affordable dementia care solutions today - while we wait for a cure!
Alzheimer's Disease and Related Dementia (ADRD) or Dementia is an umbrella term for a wide range of progressive brain diseases characterized by the onset of behavioral, cognitive, and emotional impairments primarily in older adults. The most common form of dementia is Alzheimer’s disease (60-80%), Vascular Dementia (5-10%), Lewy Body Dementia (~5%), Frontotemporal Dementia (~3%), and Parkinson's, Huntington's, and other Dementias. Alzheimer’s dementia increases with age: 5.0% of people aged 65 to 74, 13.1% of people aged 75 to 84, and 33.2% of people aged 85 and older have Alzheimer’s dementia.
01. Dementia is one of the costliest conditions in society.
An estimated 6.5M Americans ages 65 and older are living with dementia in 2022 - that’s 1 in 9 (10.9%). This number is expected to go up to 12.7M by 2050 due to the aging of the boomer generation (Alzheimer's Association). Alzheimer’s disease is the 5th leading cause of death among adults aged 65 years or older - according to the Centers for Disease Control and Prevention (CDC). In 2022, the total national cost of caring for people living with ADRD is projected to reach $321B.
To put that in perspective, the average per-person Medicare payments for healthcare and long-term care services to beneficiaries aged 65 and older with ADRD are more than 3X as great as payments for beneficiaries without these conditions, and Medicaid payments are more than 23X greater (Alzheimer's Association).
02. The financial and emotional impact on caregivers can be devastating.
More than 11 million family members and unpaid caregivers provided an estimated 16 billion hours of informal care to people with Alzheimer’s or other dementias in 2020, a contribution valued at $272B. In addition to this informal care, the out-of-pocket costs borne by families are around $81B, or 25% of the total cost of caring for people living with ADRD (Alzheimer's Association).
Nearly half of all caregivers (48%) to older adults do so for someone with Alzheimer’s or another dementia. 78% of caregivers report having out-of-pocket expenses as a result of caregiving and are spending on average a quarter of their annual income on caregiving expenses. However, caregivers caring for someone with ADRD spend 34% more money ($8,978/year with ADRD vs $6,663 with no ADRD) than those caring for someone without those conditions (AARP).
Caregiving disproportionately impacts women – two-thirds of dementia caregivers and two-thirds of dementia care recipients are women. Women caregivers also experience higher levels of burden, impaired mood, depression, and poor health than male caregivers, with evidence suggesting that these differences arise because female caregivers tend to spend more time caregiving, assume more caregiving tasks, and care for someone with more cognitive, functional and/or behavioral problems. Women are also three times as likely as men to quit their jobs to take care of a family member (AARP). This care comes at a huge personal cost to caregivers, including lost wages and benefits. According to Metlife, 10M caregivers aged 50+ who care for their parents lose an estimated $3T (that's trillion) in wages, pensions, retirement funds, and benefits.
03. Health disparity in dementia care is real.
The prevalence of ADRD varies by race and ethnicity, and eligibility for Medicare and Medicaid. The beneficiaries eligible for both Medicare and Medicaid had a higher prevalence rate (17%) than those with Medicare only (9%) (CMS). Older non-Hispanic Black Americans and Hispanic Americans are disproportionately more likely than older White Americans to have ADRD. Data from the CHAP study indicates that 19% of Black and 14% of Hispanic adults aged 65 and older have Alzheimer’s dementia compared with 10% of White older adults.
Health disparities (when a group of people experiences a higher rate of illness, injury, disability, or death than another group) can have a profound, negative effect on entire populations or individual communities. Dementia care and support services can vary widely depending on race, ethnicity, geography, and social and economic factors. Several factors lead to health disparities including stigma, cultural differences, awareness, ability to get a diagnosis, manage the disease, and be able to access quality health care. These disparities reach beyond clinical care to include uneven representation of Black, Hispanic, Asian, and Native Americans in Alzheimer’s research in clinical trials (CDC).
Whether it be virtual or in-person solutions, it's important that we are mindful of designing with health equity in mind – ensuring diversity among healthcare staff, providers who understand different racial and ethnic backgrounds, and algorithms trained to remove biases.
04. The disparity in cure versus care-related investment.
Much of the public discussion has focused, importantly, on the need for biomedical research to find preventions and treatments. In the US, less than 4% of all research was devoted to “dementia care” compared to 34% to disease pathogenesis and physiology, 16% to diagnosis, assessment and disease monitoring, and 12% to the development of therapeutics (Milken Institute). What is needed is to harness evidence-backed studies and interventions that have been discussed, trialed, and tested in academic and policy settings, and to invest in the commercialization of these programs, delivering them in real-world settings. I call this practice over policy!
05. Workforce readiness and stigma are barriers to timely detection and diagnosis.
There is a shortage of specialists such as geriatricians, neurologists, geriatric psychiatrists, and neuropsychologists who diagnose and manage treatment for people living with dementia. This shortage means that the major responsibility for diagnosing and treating people living with dementia lies with primary care physicians (PCP). However, over 50% of PCPs reported that they do not feel adequately prepared to care for individuals with Alzheimer’s and other dementias. A 2020 survey by the Alzheimer’s Association found that 82% of PCPs say they are on the front lines of providing dementia care. Even though the majority of diagnoses are made by PCPs, nearly 40% of PCPs reported never or “only sometimes or never” being comfortable personally making a diagnosis of Alzheimer’s or other dementias. A PCP visit lasts an average of 17.4 minutes, giving physicians little time to administer cognitive assessments and discuss care options on top of other services.
Stigma is also a big barrier to seeking early intervention and care. Research suggests that 50-70% of individuals at risk and their caregivers may refuse follow-up cognitive evaluation and diagnostic assessment or treatment even after screening positive, while some prefer not to know of their condition. This patient sentiment of stigma stems from the loss of a driver’s license, the loss of a home, therefore becoming dependent, and the loss of dignity. Early intervention and education of patients and their caregivers on dementia and dementia screening could increase the number of patients seeking diagnostic assessment (Milken Institute).
06. The cost of care is unsustainable.
Direct care workers, such as nurse aides/assistants, home health aides, and personal care aides, provide most of the paid long-term care to older adults living at home or in settings such as assisted living residences, nursing homes, and memory care facilities. An already acute workforce shortage is exacerbated by wage disparity, lack of training, and workplace injuries resulting in high-turnover rates and the rising cost of care.
The need and length for long-term care are likely to be exasperated for those living with Dementia. For those looking to receive care in the home, the national annual median cost for home care aide/companion in 2021 was $59.5K/$61.8K (Genworth), and average annual memory care facility costs in 2021 were $70K (AARP). By 2030 the national annual median costs are expected to see a 30% increase compared to those in 2021. According to the HHS, 70% of people over the age of 65 will need some type of long-term care support - an expense not covered under Medicare, and unfortunately, few have saved enough money to pay for outside help during their retirement.
07. CMS moves the needle on reimbursement to encourage cognitive assessment.
CMS has attempted to boost the utilization of cognitive assessments in routine care by making them a requirement of the Medicare Annual Wellness Visit, reimbursing the cognitive assessment and care plan as a separate visit and at a higher dollar amount, and making the option of reimbursable telehealth evaluation permanent.
Additionally, in 2020 CMS reintroduced ADRD HCCs (Hierarchical Condition Category) to risk-adjust Medicare Advantage (MA) and Accountable Care Organization (ACO) payments. Risk adjustment is a statistical method that predicts a person’s likely use and costs of health care services and assigns a risk score to each beneficiary based on their diagnoses and demographic characteristics. It’s used in MA to adjust the capitated payments the federal government makes to cover the expected medical costs of enrollees.
ADRD is currently underdiagnosed, and the inclusion of these conditions in the HCC risk adjustment model represents an opportunity and financial incentive for MA plans to capture the relevant diagnosis codes. These additional risk-adjusted, capitated payments go to health plans, and the hope is that beneficiaries also derive benefits with access to cognitive screening, quality of care, reduced premiums, and out-of-pocket costs. Higher rebates to MA plans also enable them to offer extra supplemental benefits (Commonwealth Fund, Milliman).
08. The long game is comprehensive, coordinated care that bridges clinical and social support.
Proper care for those diagnosed with dementia requires better coordination of care, seamless navigation across the multitude of providers, timely access to care and interventions, and support for caregivers. Over the last decade, the Center for Medicare and Medicaid Innovation (CMMI) funded dementia care management pilot projects, including the UCLA Alzheimer's and Dementia Care (ADC) program, the UCSF Care Ecosystem, and Eskenazi Health and Indiana University's Aging Brain Care (ABC) program. These models demonstrated that managing the care of people with dementia can reduce hospitalizations and emergency department visits and delay nursing home placement, thus improving outcomes and reducing total costs.
Despite their success, dementia care management programs operating under the Medicare fee-for-service (FFS) system do not provide reimbursement for many of the patient and caregiver services that are key to the program's success. Bottom line - the transition from a fee-for-service payment structure to a capitated fee payment model is necessary to pay for care coordination and home and community-based services. A proposal by the Alzheimer's Association uses the delivery framework of the dementia care management pilot programs previously funded by CMMI and creates a capitated and performance-based Alternate Payment Model (APM) structure for reimbursing providers. If approved, it could pave the path for scaling these solutions and go beyond current academic centers, making them available to dementia patients across the Medicare population through MA plans and Integrated Delivery Networks. An analysis commissioned by Alzheimer’s Impact Movement found that dementia care management would save Medicare and Medicaid nearly $21B over 10 years, and the federal government would see a 2.6:1 rate of return.
Market innovation in dementia care.
While we wait for payment reforms, millions of people living with dementia and their caregivers do not receive high-quality, coordinated care that provides medical, neuropsychological, and social services. Providing long-term care support services like assistance with home care and other activities of daily living falls almost entirely on the shoulders of financially and emotionally burdened caregivers. We have seen a growing number of companies focused on dementia care – from novel cognitive assessments to digital therapeutics to support families in need, while we wait for a cure.
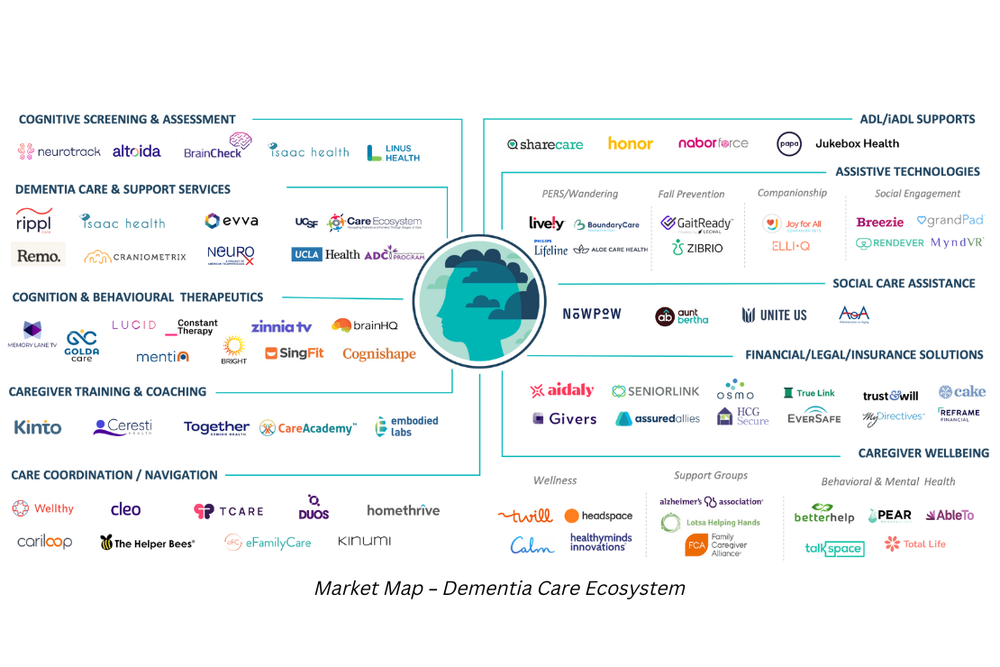
Four key takeaways for entrepreneurs.
Dementia remains one of the toughest challenges in healthcare. While there is no cure for dementia, new treatments aim to slow cognitive decline and manage symptoms. People living with dementia are likely to need a combination of drug treatments, clinical care, and support services to help them live their fullest lives. There are a few areas that are ripe for entrepreneurs.
1. “How might we – accelerate the commercialization of evidence-based studies?
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) is the first randomized controlled trial showing that it is possible to prevent cognitive decline using a multidomain lifestyle intervention among older at-risk individuals. The intervention includes nutritional guidance, physical exercise, cognitive training, social activities, and vascular and metabolic risk factors management. Results from this trial showed a 25% increase in overall cognition and even greater gains in individual domains such as memory and processing speed. The intervention also showed a 60% decrease in other chronic diseases (FINGERS). With such promising evidence, we need to accelerate the commercialization of its use in Medicare and Medicaid, similar to lifestyle modification programs in Diabetes and Cardiovascular Health in employer-sponsored benefits.
2. “How might we – think about a virtual dementia care model?”
Given the acute shortage of dementia specialists and direct care workforce and the challenges with PCPs in taking on dementia care, it would seem logical to look at virtual care models to address the challenges of supply shortage, and also drive efficiencies of using telehealth and other digital technologies to improve access to diagnosis and managing care. A virtual memory clinic of sorts could administer cognitive assessments, provide ongoing care planning, conduct ongoing monitoring, do medication management, implement psychosocial interventions, and educate/train family caregivers. While some of these are reimbursable, it presents an opportunity to support stressed caregivers with an integrated dementia model of care at home.
3. "How might we – help the caregiver better manage dementia-related behaviors?”
Behavioral and psychological symptoms of dementia, also known as neuropsychiatric symptoms, affect up to 90% of all dementia subjects over the course of their illness, and these are as clinically relevant as cognitive symptoms. These behaviors include agitation, aberrant motor behavior, anxiety, elation, irritability, depression, apathy, disinhibition, delusions, hallucinations, and sleep or appetite changes. Although these symptoms can be present individually, it is more common that they occur simultaneously in a patient (Frontiers in Neurology, Alzheimer’s Association). A research study found the prevalence of agitation was 61.3% among patients with staged ADRD, with the highest in moderate‐to‐severe stages (74.6% and 68.3%, respectively). Underlying medical conditions, unmet needs, environmental triggers, and some medications can cause these behavioral symptoms or make them worse. These symptoms are complex and costly aspects of care, leading to poor patient health outcomes, and stress to family caregivers.
How might we use technology to detect and predict the onset of such behaviors and support family caregivers with timely interventions to calm their loved ones through non-pharmacologic interventions such as calming audio-visual content, music therapy, reminiscence therapy, and activities?
4. “How might we – rethink the MA benefit design for dementia?”
In 2018, CMS provided guidance that allowed MA plans to offer supplemental benefits targeting specific disease states as long as “similarly situated individuals are treated uniformly.” This allows Medicare Advantage plans to reduce cost-sharing for certain covered benefits (e.g., offering enrollees a lower deductible) or to tailor supplemental benefits for enrollees who meet specific medical criteria (e.g., “Personal Emergency Response Systems (PERS) for enrollees at high risk of falls”), as long as all enrollees who meet the identified criteria receive the same access to these targeted benefits. In 2022, dementia is targeted by only 165 plans with additional benefits (and 0 plans with reduced cost sharing) out of a total of 3,834 plans nationally (Milliman, KFF). The new HCC risk adjustment reforms also provide financial incentives for MA plans to offer extra support to those living with dementia. Given the high out-of-pocket costs associated with long-term care at home, how might we rethink how MA plans could provide more flexibility and meaningful benefits to support beneficiaries and their caregivers?
Call for innovation.
It’s time we accelerate technical innovation and evidence-based approaches into the hands of caregivers and people living with dementia – moving from policy to practice. While by no means exhaustive, there are three broad areas of innovation that are needed to bring viable solutions to market scale.
1. Improved access to non-invasive cognitive screening: Digital and interactive versions of pen-paper tests to de novo approaches that use noninvasive biomarkers such as analyzing blood, retinal scans, or voice patterns are essential to improving access. These tests are designed to be administered easily in a primary care physician's office, academic medical centers, community, and eventually in home settings. These tech-enabled, non-invasive screening tools that are integrated into the clinical workflow and engage family caregivers are critical to early detection by lowering the barrier to access. This is the “top of the screening funnel.”
2. Building workforce capacity: A dementia-capable workforce that is well-trained is going to be critical in the long run to address the complex and highly variable needs of people living with dementia - from a PCP being able to have the “now what?” conversation with caregivers, and for caregivers, formal and informal, being trained to tackle the behavioral challenges that come with caring for someone with dementia. Solutions that provide decision support to clinicians, those that tackle the challenges of reducing the turnover of direct care workers, and upskilling informal family caregivers build resilience in the care ecosystem. We must invest in “Care Resilience” infrastructure.
3. Integrating technology in the care continuum: Virtual memory clinic models could provide cognitive assessments, care planning, and ongoing cognitive monitoring. This would start to address the challenges of access and supply shortage. This telehealth model can operate in current FFS environments while we await reforms to move into comprehensive, value-based care. Let's not let perfection get in the way of practicality. Additionally, the use of in-home technology to passively monitor people with dementia remotely can reduce or potentially eliminate the use of emergency services and expensive hospital visits. These range from assistive technologies that detect wandering and prevent falls, to digital therapeutics that detect and manage neuropsychiatric symptoms of dementia such as agitation and others. We must leverage virtual care and technology to make “care more accessible and practical for families.”
Dementia remains one of the toughest challenges in healthcare. Proper and effective care requires early diagnosis, better coordination of care, and timely access to care and interventions. The reality is that long-term care for someone with dementia falls entirely on families – physically, emotionally, and financially, and it comes at a huge personal cost to caregivers, including lost wages and benefits. As a health tech industry, we need to see more investments and commercialization of innovative care models based on evidence-backed research. We hope that the smart use of technology can bend the curve by improving access to screening and most importantly assisting caregivers in monitoring and management tools to improve the quality of life for those living with dementia.
About Techstars
Techstars invests in early-stage startups led by unstoppable entrepreneurs with transformative businesses. With 45+ accelerators worldwide, an unrivaled network of alumni, mentors, commercial partners, investors, and dedicated operating teams, Techstars supports entrepreneurs throughout their entire startup journey while helping to build thriving startup communities. Since 2006, we have invested in more than 4,400 portfolio companies, accelerating the growth of businesses including Chainalysis, Zipline, DataRobot, Alloy, and many, many more. www.techstars.com
About Pivotal Ventures
Pivotal Ventures, a Melinda French Gates company, accelerates social progress in the United States. We remove barriers to equality and opportunity through investments, partnerships, and advocacy.
About the Author

Sanjay Khurana
Sanjay spent his career building and delivering innovative products and delightful consumer experiences at companies from Fortune 5 to tech start-ups. He has held senior leadership roles in product development, innovation, strategy, and new business ventures development in digital health, consumer technologies, media and communications. Previously he's held positions at CVS Health, as Vice President of Caregiving Products and Services at AARP, and Director of Innovation and Thought Leadership at AARP.
